F.D.A. Approves Alzheimer’s Drug Despite Fierce Debate Over Whether It Works
Aducanumab is the first new Alzheimer’s treatment in 18 years and the first to attack the disease process. But some prominent experts say there’s not enough evidence it can address cognitive symptoms.
The Food and Drug Administration on Monday approved the first new medication for Alzheimer’s disease in nearly two decades, a contentious decision, made despite opposition from the agency’s independent advisory committee and some Alzheimer’s experts who said there was not enough evidence that the drug can help patients.
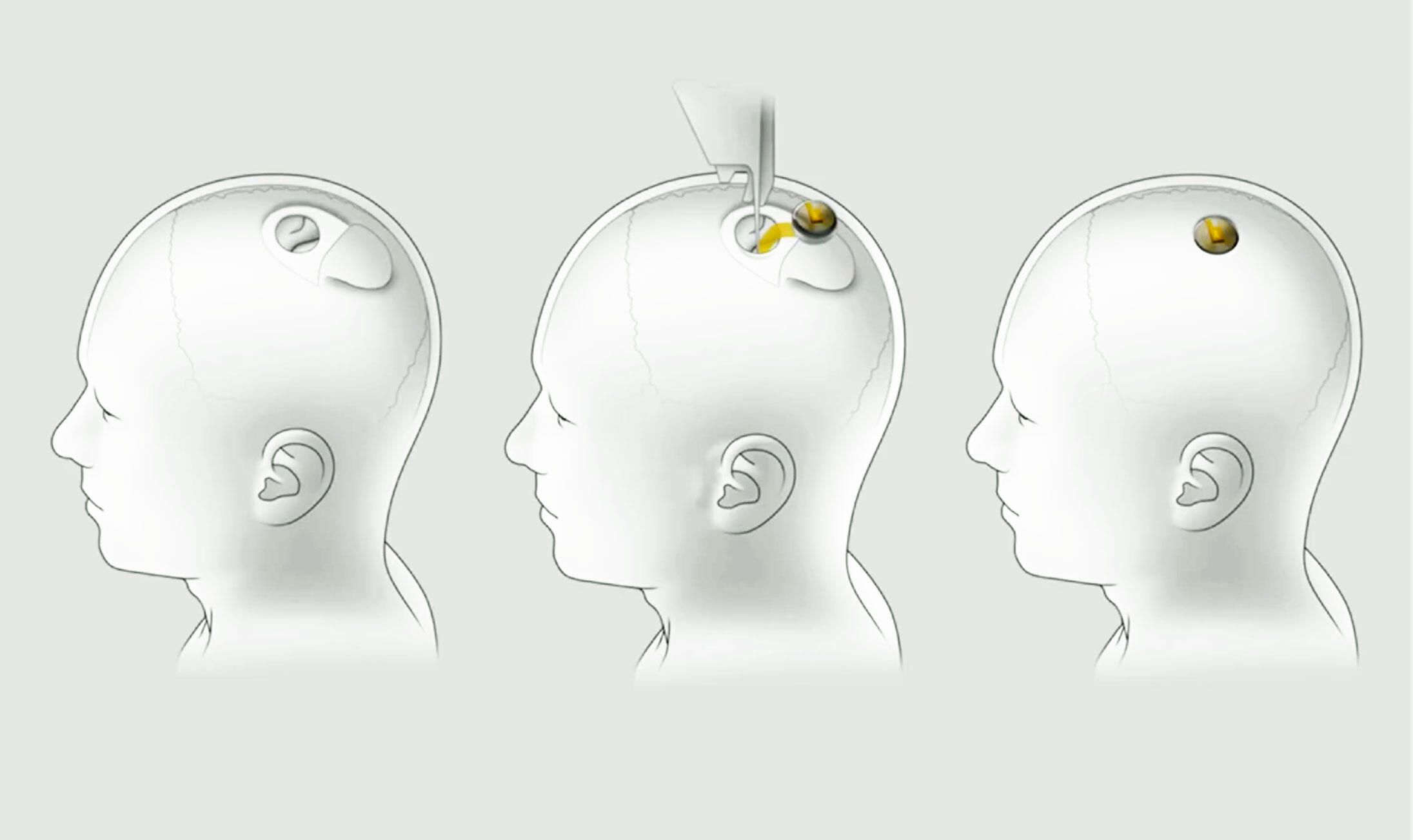
The drug, aducanumab, which will go by the brand name Aduhelm, is a monthly intravenous infusion intended to slow cognitive decline in people, with mild memory and thinking problems. It is the first approved treatment to attack the disease process of Alzheimer’s instead of just addressing dementia symptoms.
Recognizing that clinical trials of the drug had provided incomplete evidence to demonstrate effectiveness, the F.D.A. granted approval on the condition that the manufacturer, Biogen, conduct a new clinical trial.










:quality(70)/cloudfront-us-east-1.images.arcpublishing.com/archetype/CTKYCDUFNBE4NPJAVZFAZHVNCE.jpg)