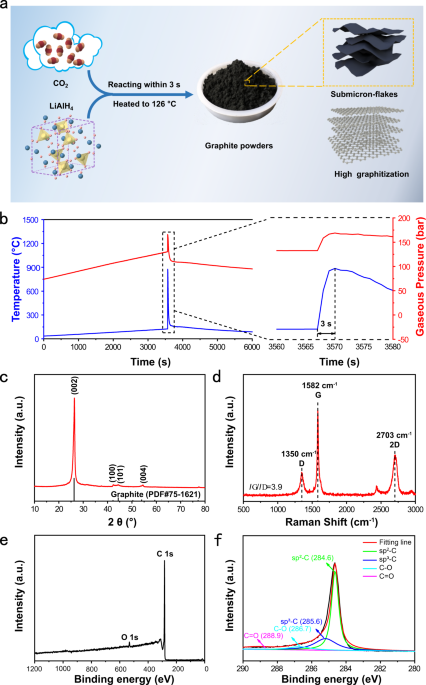
Green synthesis of graphite from CO2 without graphitization process of amorphous carbon
Nature Communications volume 12, Article number: 119 (2021 ) Cite this article
Environmentally benign synthesis of graphite at low temperatures is a great challenge in the absence of transition metal catalysts. Herein, we report a green and efficient approach of synthesizing graphite from carbon dioxide at ultralow temperatures in the absence of transition metal catalysts. Carbon dioxide is converted into graphite submicroflakes in the seconds timescale via reacting with lithium aluminum hydride as the mixture of carbon dioxide and lithium aluminum hydride is heated to as low as 126 °C. Gas pressure-dependent kinetic barriers for synthesizing graphite is demonstrated to be the major reason for our synthesis of graphite without the graphitization process of amorphous carbon. When serving as lithium storage materials, graphite submicroflakes exhibit excellent rate capability and cycling performance with a reversible capacity of ~320 mAh g–1 after 1500 cycles at 1.0 A g–1. This study provides an avenue to synthesize graphite from greenhouse gases at low temperatures.
Elemental carbon has been extensively applied in the interdisciplinary fields spanning catalysis, metallurgy, environmental remediation, energy storage and conversion, automotive industry, and drug delivery because of its tunable physicochemical properties1,2,3,4,5,6. Although carbon is the fourth most abundant element in nature by mass, more than 99% of carbon appears in the form of compounds such as metal carbonates, organics, carbides, and carbon dioxide/monoxide7,8,9,10,11,12. The controllable synthesis of elemental carbon from carbon containing compounds has become the major strategy of achieving carbon materials with various physicochemical properties7,8,9,10,13,14. Carbon atoms bond together in different ways to form carbon allotropes with different physicochemical properties. Graphite, the most thermodynamically stable allotrope under standard conditions, has attracted special attention owing to its excellent physicochemical properties, including electrochemical lithium storage, electric and thermal conduction, superlubricity, and chemical and thermo stability7,15,16,17.




















/cdn.vox-cdn.com/uploads/chorus_asset/file/25419483/247092_Student_activist_doxxing_AKrales_1438.jpg)