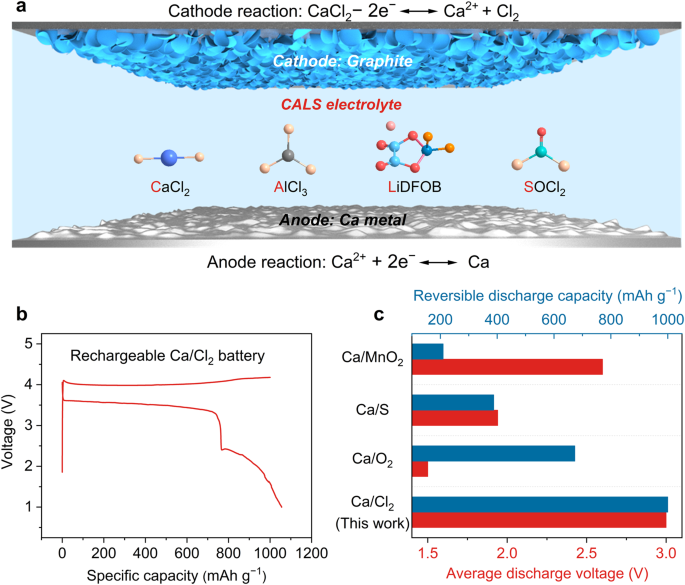
A rechargeable Ca/Cl2 battery
Nature Communications volume 15, Article number: 944 (2024 ) Cite this article
Rechargeable calcium (Ca) metal batteries are promising candidates for sustainable energy storage due to the abundance of Ca in Earth’s crust and the advantageous theoretical capacity and voltage of these batteries. However, the development of practical Ca metal batteries has been severely hampered by the current cathode chemistries, which limit the available energy and power densities, as well as their insufficient capacity retention and low-temperature capability. Here, we describe the rechargeable Ca/Cl2 battery based on a reversible cathode redox reaction between CaCl2 and Cl2, which is enabled by the use of lithium difluoro(oxalate)borate as a key electrolyte mediator to facilitate the dissociation and distribution of Cl-based species and Ca2+. Our rechargeable Ca/Cl2 battery can deliver discharge voltages of 3 V and exhibits remarkable specific capacity (1000 mAh g−1) and rate capability (500 mA g−1). In addition, the excellent capacity retention (96.5% after 30 days) and low-temperature capability (down to 0 °C) allow us to overcome the long-standing bottleneck of rechargeable Ca metal batteries.
Modern electrification has witnessed the ever-growing demand for rechargeable batteries with high sustainability and energy storage capabilities1,2,3,4. Rechargeable calcium (Ca) metal batteries are among the most promising candidates because of their advantageous features, such as high crustal abundance, high theoretical capacity, and ideal redox potential5,6,7. However, compared with the conventional Li counterparts, current Ca metal batteries based on intercalation/deintercalation cathodes, such as metal oxides, suffer from relatively limited electrochemical performance, e.g., low specific capacities (<200 mAh g−1), discharge voltages (<2.6 V), and rate capability (<100 mA g−1)8,9,10,11, which requires the development of new cathode reactions to overcome these limitations on electrochemical performance. In addition, the capacity retention and low-temperature performance of Ca metal batteries remain inaccessible to date, due to the inferior electrochemical stability and sluggish cathode/anode reaction kinetics. Therefore, it is crucial yet challenging to develop new cathode chemistry to realize practical applications of rechargeable Ca metal batteries12,13,14.










/cdn.vox-cdn.com/uploads/chorus_asset/file/25446024/maxresdefault__2_.jpg)
/https%3A%2F%2Ftf-cmsv2-smithsonianmag-media.s3.amazonaws.com%2Ffiler_public%2Fd2%2F47%2Fd247f070-e85b-467e-a486-b16cc64091d3%2F23_0159_smithsonian_scanner_m_w3-cc1-3k.jpg)