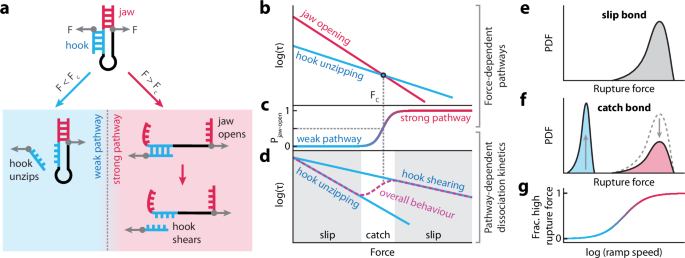
Engineering tunable catch bonds with DNA
Nature Communications volume 15, Article number: 8828 (2024 ) Cite this article
Unlike most adhesive bonds, biological catch bonds strengthen with increased tension. This characteristic is essential to specific receptor-ligand interactions, underpinning biological adhesion dynamics, cell communication, and mechanosensing. While artificial catch bonds have been conceived, the tunability of their catch behaviour is limited. Here, we present the fish-hook, a rationally designed DNA catch bond that can be finely adjusted to a wide range of catch behaviours. We develop models to design these DNA structures and experimentally validate different catch behaviours by single-molecule force spectroscopy. The fish-hook architecture supports a vast sequence-dependent behaviour space, making it a valuable tool for reprogramming biological interactions and engineering force-strengthening materials.
Cells communicate mechanically by applying tension across cell-cell and cell-environment adhesions1,2,3,4,5. The transduction of specific signals across these adhesive junctions relies on the force-dependent behaviour of the receptors and ligands. Many biochemical pathways activate as a response to external force, and the degree of this activation can vary depending on the magnitude, direction, loading period, and loading rate of mechanical strain. For example, T-cell receptor (TCR) specificity between agonists and antagonists is enhanced 4-fold when tension is applied6, focal adhesion maturation is mediated by sustained high forces and fast loading rates7, cell polarization during migration is determined by direction-dependent tension between vinculin and actin8, and E. coli avoids being flushed out by the high shear stress of urinal and intestinal tracts while retaining mobility under low shear stress9. In all these cases, measuring the dissociation constant (Kd) or lifetime in a zero-force environment (τ0) is not enough to see the full scale of biologically relevant behaviours.