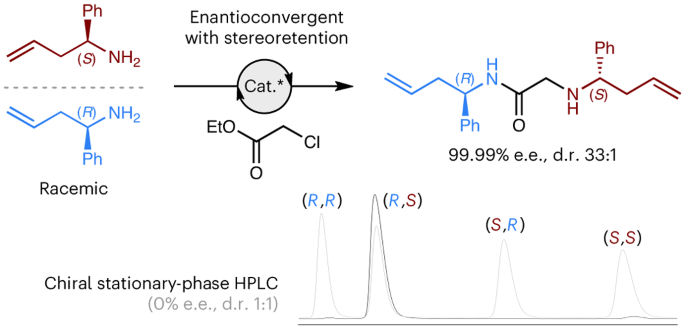
Stereoretentive enantioconvergent reactions
Enantioconvergent reactions are pre-eminent in contemporary asymmetric synthesis as they convert both enantiomers of a racemic starting material into a single enantioenriched product, thus avoiding the maximum 50% yield associated with resolutions. All currently known enantioconvergent processes necessitate the loss or partial loss of the racemic substrate’s stereochemical information, thus limiting the potential substrate scope to molecules that contain labile stereogenic units. Here we present an alternative approach to enantioconvergent reactions that can proceed with full retention of the racemic substrate’s configuration. This uniquely stereo-economic approach is possible if the two enantiomers of a racemic starting material are joined together to form one enantiomer of a non-meso product. Experimental validation of this concept is presented using two distinct strategies: (1) a direct asymmetric coupling approach, and (2) a multicomponent approach, which exhibits statistical amplification of enantiopurity. Thus, the established dogma that enantioconvergent reactions require substrates that contain labile stereogenic units is shown to be incorrect.
From medicine to materials science1,2, the ability to control the absolute configuration of chiral molecules is vital to controlling their function (Fig. 1a). Since Pasteur’s seminal work on the chiral resolution of racemic tartrates3, scientists have sought out new ways to access chiral molecules in enantioenriched form. The stereoselective synthesis of chiral molecules in enantioenriched form, known as asymmetric synthesis, has been a great success for the discipline of synthetic organic chemistry. The importance of asymmetric synthesis was recognized in 2001 when the Nobel Prize in Chemistry was awarded to Knowles, Noyori and Sharpless for their development of metal-catalysed asymmetric reactions. The field has remained an innovative and vibrant area of research, with the 2021 Nobel Prize in Chemistry awarded to List and MacMillan for their development of organocatalysed asymmetric reactions. Indeed, asymmetric reactions are now routine, both in industrial and academic settings, with a wide variety of catalysts available, from intricate precious-metal complexes to bespoke engineered enzymes.