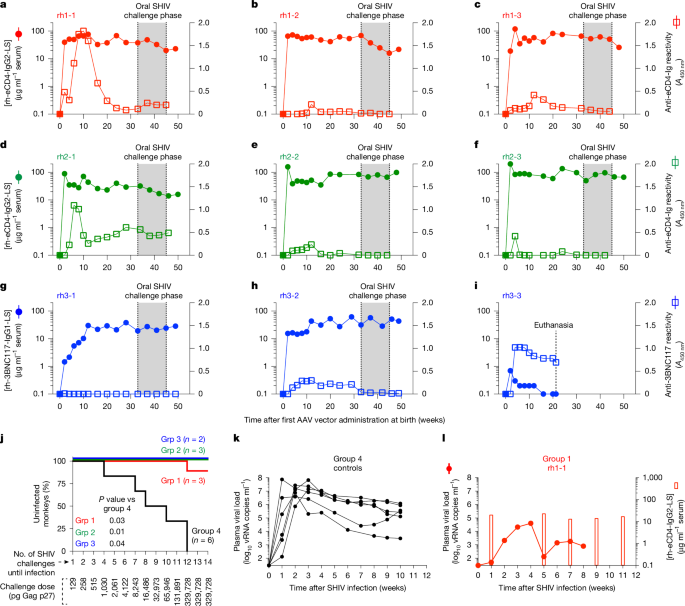
Determinants of successful AAV-vectored delivery of HIV-1 bNAbs in early life
Despite advances in HIV-1 prophylaxis, vertical transmission remains a pressing problem in developing countries1. Given the promise of broadly neutralizing antibodies (bNAbs) for HIV-1 prevention2, we hypothesized that neonatal delivery of bNAbs using adeno-associated virus (AAV) could provide durable HIV-1 immunity during infancy. Here, using infant rhesus macaques (Macaca mulatta) as a model, we show that a one-time administration of an AAV vector encoding bNAb 3BNC117 at birth led to sustained bNAb expression for more than three years without redosing. This approach significantly protected both infant and pre-adolescent rhesus macaques from infection with simian–human immunodeficiency virus in mucosal challenge models that mimic HIV-1 transmission through breastfeeding and sexual intercourse. Age at the time of AAV-3BNC117 administration was a main determinant of success and was inversely correlated with the incidence of host anti-drug antibodies that restricted bNAb expression. Consistent with principles of neonatal tolerance3,4, newborn rhesus macaques exhibited higher levels of bNAb expression than older infants and juveniles following AAV-3BNC117 dosing. Furthermore, in utero exposure to recombinant 3BNC117 suppressed anti-drug antibodies and improved AAV-vectored delivery of this bNAb in older infants. Thus, our results suggest that neonatal and fetal immunological tolerance can be leveraged to improve postnatal AAV delivery of HIV-1 bNAbs in primates. Since years-long HIV-1 immunity can be generated in rhesus macaques from a one-time AAV vector administration at birth, future studies should evaluate the ability of this strategy to prevent perinatal and adolescent HIV-1 infections in humans.
Every year, more than 100,000 children acquire HIV-1, with the majority of cases occurring in sub-Saharan Africa due to vertical transmission1. Complex social and economic barriers continue to hinder efforts to eliminate perinatal HIV-1 transmission in the region, with gender power imbalances having a key role5. Many women lack the ability to negotiate condom use with their male partners, leaving them vulnerable to HIV-1 and unintended pregnancies. Compounding these issues, only half of pregnant and breastfeeding women who are living with HIV-1 in western and central Africa were receiving antiretroviral (ARV) therapy in 20231. These regions now account for 41% of all paediatric HIV-1 infections worldwide1. Adherence to ARV therapy often declines postpartum, particularly among young mothers who disengage from care after delivery1. Treatment lapses increase maternal viraemia and the risk of transmission through breastfeeding. Meanwhile, paediatric formulations of ARV therapies, which are used for both prophylaxis and therapy, are limited, difficult to administer and undermined by drug toxicity and resistance6,7. Although long-acting ARV therapies may improve patient compliance, their use in infants remains unproven and constrained by access8.