/cloudfront-us-east-2.images.arcpublishing.com/reuters/3G7J5D46XBK3BILS7SSGMG6KA4.jpg)
CureVac fails in pivotal COVID-19 vaccine trial with 47% efficacy
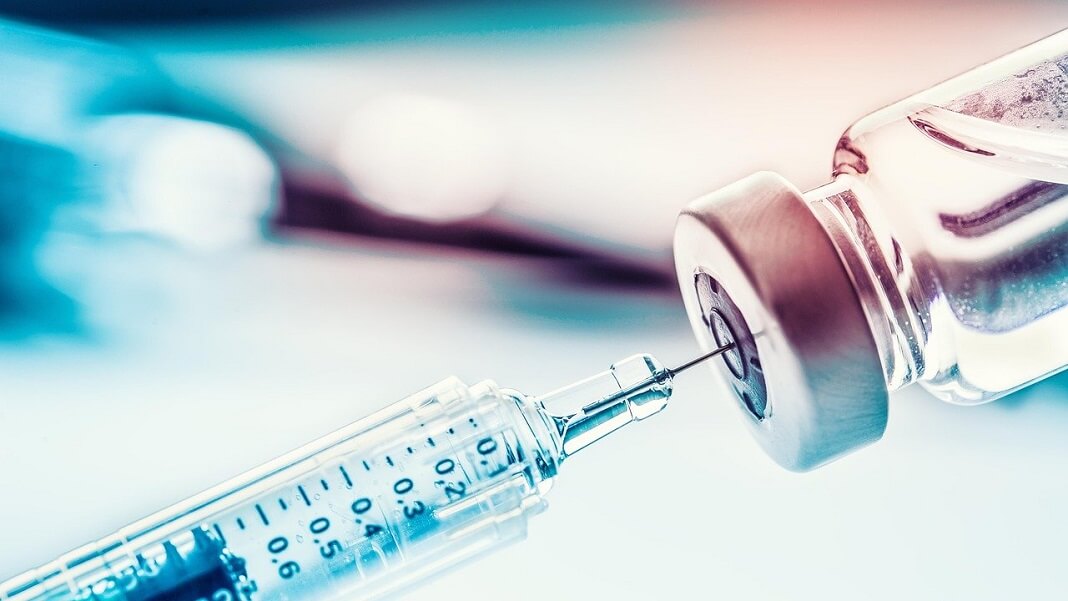
A vaccination against the coronavirus disease (COVID-19) from German biotechnology company CureVac is ready to use at the start of a clinical test series in the tropical institute of the university clinic in Tuebingen, Germany, June 22, 2020. REUTERS/Kai Pfaffenbach
June 16 (Reuters) - German biotech CureVac NV (5CV.DE) said on Wednesday its COVID-19 vaccine was only 47% effective in a late-stage trial, missing the study’s main goal and throwing in doubt the potential delivery of hundreds of millions of doses to the European Union.
The disappointing efficacy of the shot known as CVnCoV emerged from an interim analysis based on 134 COVID-19 cases in the study with about 40,000 volunteers in Europe and Latin America.
The stakes for CureVac and prospective buyers of its vaccine in Europe had risen after age limits were imposed on the use of the Johnson & Johnson (JNJ.N) and AstraZeneca (AZN.L) vaccines due to a link to extremely rare but potentially fatal clotting disorders.
CureVac's shot was also expected to help in low and middle-income countries that have lagged far behind richer nations in the global immunisation drive.
Leave a Comment
Related Posts
Israel says Pfizer Covid vaccine is just 39% effective as delta spreads, but still prevents severe illness
Comment