Scientists engineer CRISPR enzymes that evade the immune system
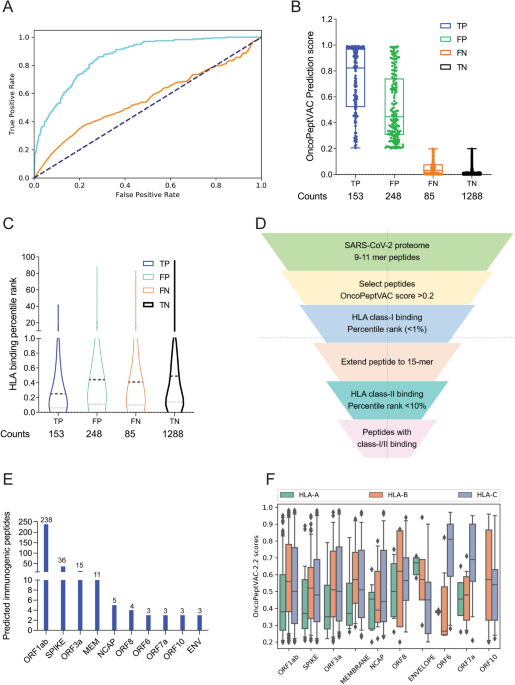
The core components of CRISPR-based genome-editing therapies are bacterial proteins called nucleases that can stimulate unwanted immune responses in people, increasing the chances of side effects and making these therapies potentially less effective.
Researchers at the Broad Institute of MIT and Harvard and Cyrus Biotechnology have now engineered two CRISPR nucleases, Cas9 and Cas12, to mask them from the immune system. The team identified protein sequences on each nuclease that trigger the immune system and used computational modeling to design new versions that evade immune recognition. The engineered enzymes had similar gene-editing efficiency and reduced immune responses compared to standard nucleases in mice.
Appearing today in Nature Communications, the findings could help pave the way for safer, more efficient gene therapies. The study was led by Feng Zhang, a core institute member at the Broad and an Investigator at the McGovern Institute for Brain Research at MIT.
“As CRISPR therapies enter the clinic, there is a growing need to ensure that these tools are as safe as possible, and this work tackles one aspect of that challenge,” said Zhang, who is also a co-director of the K. Lisa Yang and Hock E. Tan Center for Molecular Therapeutics, the James and Patricia Poitras Professor of Neuroscience, and a professor at MIT. He is an Investigator at the Howard Hughes Medical Institute.
Leave a Comment
Related Posts