High-throughput identification of repurposable neuroactive drugs with potent anti-glioblastoma activity
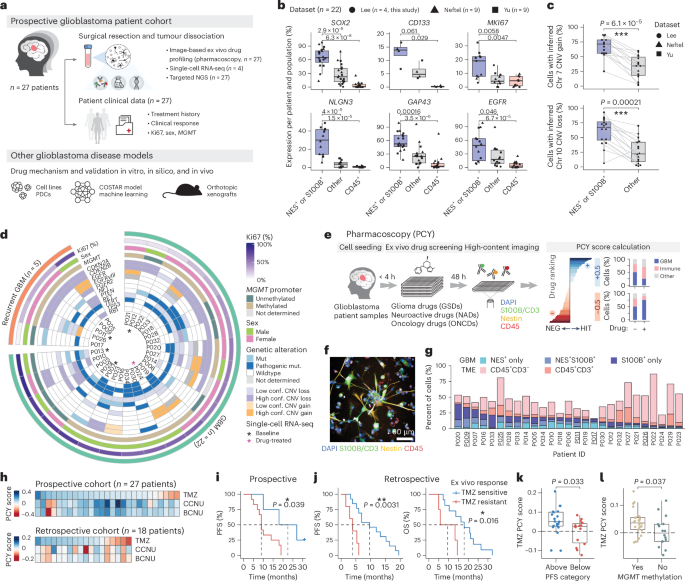
Glioblastoma, the most aggressive primary brain cancer, has a dismal prognosis, yet systemic treatment is limited to DNA-alkylating chemotherapies. New therapeutic strategies may emerge from exploring neurodevelopmental and neurophysiological vulnerabilities of glioblastoma. To this end, we systematically screened repurposable neuroactive drugs in glioblastoma patient surgery material using a clinically concordant and single-cell resolved platform. Profiling more than 2,500 ex vivo drug responses across 27 patients and 132 drugs identified class-diverse neuroactive drugs with potent anti-glioblastoma efficacy that were validated across model systems. Interpretable molecular machine learning of drug–target networks revealed neuroactive convergence on AP-1/BTG-driven glioblastoma suppression, enabling expanded in silico screening of more than 1 million compounds with high patient validation accuracy. Deep multimodal profiling confirmed Ca2+-driven AP-1/BTG-pathway induction as a neuro-oncological glioblastoma vulnerability, epitomized by the anti-depressant vortioxetine synergizing with current standard-of-care chemotherapies in vivo. These findings establish an actionable framework for glioblastoma treatment rooted in its neural etiology.
Glioblastoma is the deadliest primary brain cancer with limited treatment options, shaped by heterogeneous developmental programs, genetic drivers and tumor microenvironments (TMEs)1,2,3,4,5,6. Despite an increasing understanding of this heterogeneity, the alkylating agent temozolomide (TMZ), prolonging median survival from 12 months to 15 months, remains the only first-line drug approved for glioblastoma7,8. Targeted therapies have been largely unsuccessful, in part due to the blood–brain barrier (BBB) limiting tumor accessibility, the presence of treatment-resistant glioblastoma stem cells (GSCs) and the lack of clinically predictive patient model systems9,10,11. Systemically addressing these therapeutic roadblocks is an urgent clinical need.
Leave a Comment
Related Posts