FDA approves breakthrough eye drops that fix near vision without glasses
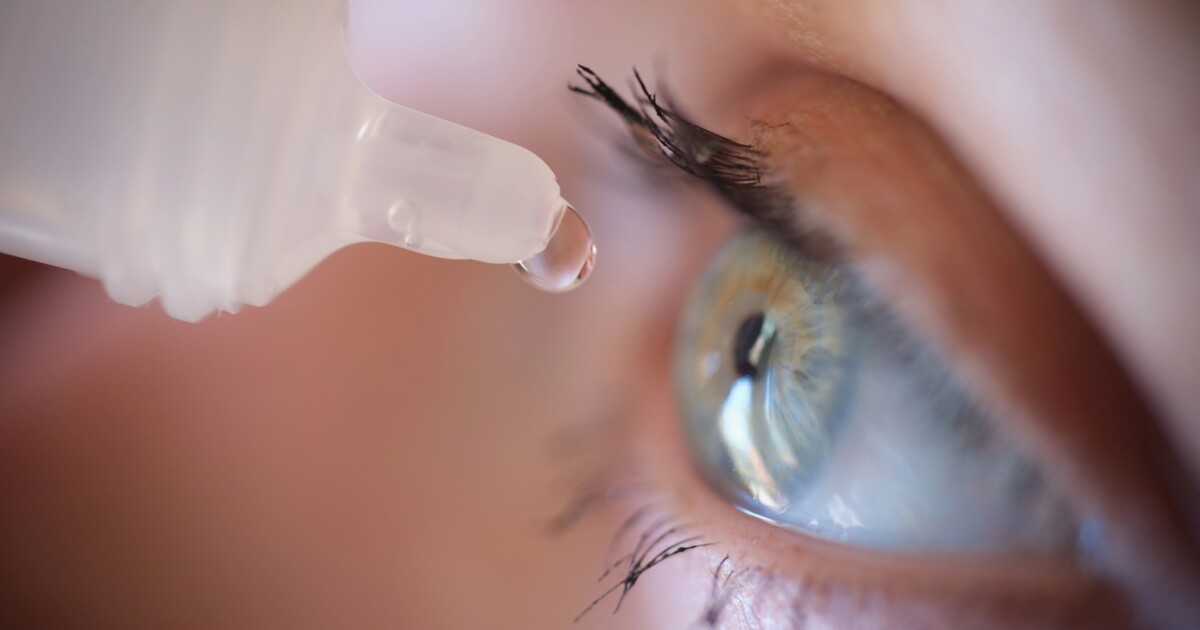
The first aceclidine-based eye drop to improve near vision in adults with presbyopia, which affects more than 100 million adults in the US alone, has been approved by the Food and Drug Administration (FDA) and will be available within three months.
Known as VIZZ, from pharmaceutical company LENZ, the drops are an aceclidine ophthalmic solution that effectively treats presbyopia in adults. The once-daily drops offer relief from blurry near. vision for up to 10 hours.
"The FDA approval of VIZZ is a defining moment for LENZ and represents a transformative improvement in the available treatment options for the 128 million adults living with blurry near vision in the United States," said Eef Schimmelpennink, President and Chief Executive Officer of LENZ Therapeutics. "This significant milestone is the result of tremendous commitment and collaboration by the LENZ team and our partners, the dedication of our clinical investigators, and the contributions of hundreds of participants in our clinical trials."
VIZZ works by gently shrinking the pupil of the eye, using aceclidine. This creates a “pinhole effect” – like narrowing a camera lens — which helps bring nearby objects into sharper focus. Unlike older eye drops, this one does not significantly affect the eye’s focusing muscles, so it doesn’t blur your distance vision or cause that “zoomed-in” effect (aka a myopic shift).