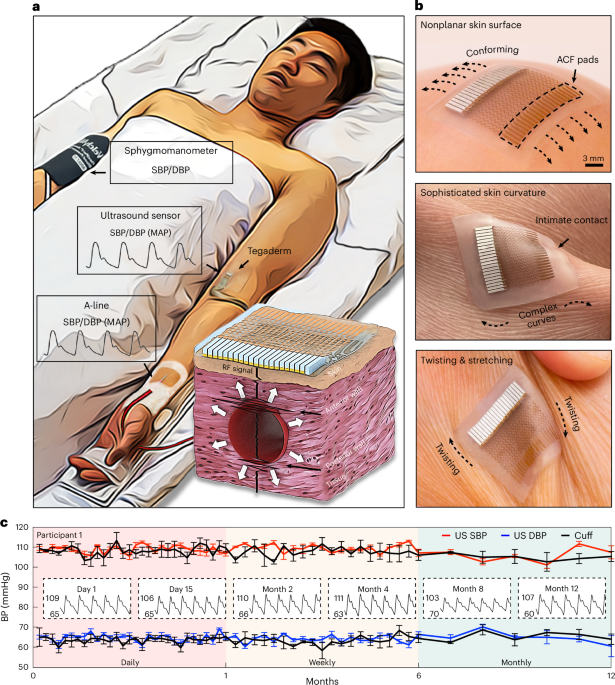
Clinical validation of a wearable ultrasound sensor of blood pressure
Nature Biomedical Engineering (2024 )Cite this article
Options for the continuous and non-invasive monitoring of blood pressure are limited. Cuff-based sphygmomanometers are widely available, yet provide only discrete measurements. The clinical gold-standard approach for the continuous monitoring of blood pressure requires an arterial line, which is too invasive for routine use. Wearable ultrasound for the continuous and non-invasive monitoring of blood pressure promises to elevate the quality of patient care, yet the isolated sonographic windows in the most advanced prototypes can lead to inaccurate or error-prone measurements, and the safety and performance of these devices have not been thoroughly evaluated. Here we describe validation studies, conducted during daily activities at home, in the outpatient clinic, in the cardiac catheterization laboratory and in the intensive care unit, of the safety and performance of a wearable ultrasound sensor for blood pressure monitoring. The sensor has closely connected sonographic windows and a backing layer that improves the sensor’s accuracy and reliability to meet the highest requirements of clinical standards. The validation results support the clinical use of the sensor.
The main data supporting the results in this study are available within the paper and its Supplementary Information. Source data for the figures are available from figshare at https://doi.org/10.6084/m9.figshare.25511761 (ref. 95).












/cdn.vox-cdn.com/uploads/chorus_asset/file/25749641/2165011649.jpg)



