Formation of memory assemblies through the DNA-sensing TLR9 pathway
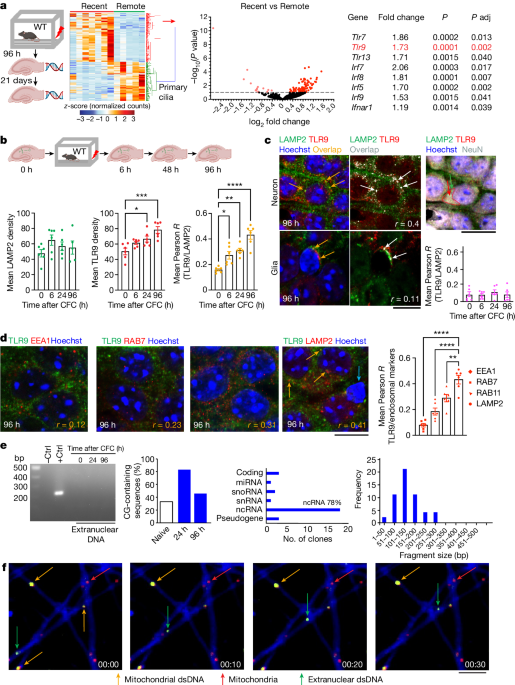
As hippocampal neurons respond to diverse types of information1, a subset assembles into microcircuits representing a memory2. Those neurons typically undergo energy-intensive molecular adaptations, occasionally resulting in transient DNA damage3,4,5. Here we found discrete clusters of excitatory hippocampal CA1 neurons with persistent double-stranded DNA (dsDNA) breaks, nuclear envelope ruptures and perinuclear release of histone and dsDNA fragments hours after learning. Following these early events, some neurons acquired an inflammatory phenotype involving activation of TLR9 signalling and accumulation of centrosomal DNA damage repair complexes6. Neuron-specific knockdown of Tlr9 impaired memory while blunting contextual fear conditioning-induced changes of gene expression in specific clusters of excitatory CA1 neurons. Notably, TLR9 had an essential role in centrosome function, including DNA damage repair, ciliogenesis and build-up of perineuronal nets. We demonstrate a novel cascade of learning-induced molecular events in discrete neuronal clusters undergoing dsDNA damage and TLR9-mediated repair, resulting in their recruitment to memory circuits. With compromised TLR9 function, this fundamental memory mechanism becomes a gateway to genomic instability and cognitive impairments implicated in accelerated senescence, psychiatric disorders and neurodegenerative disorders. Maintaining the integrity of TLR9 inflammatory signalling thus emerges as a promising preventive strategy for neurocognitive deficits.
Memories of individuals’ experiences are represented across assemblies of neurons in hippocampal and cortical circuits. Several mechanisms of formation and maintenance of these assemblies have been proposed. The most prominent such mechanism is stimulus-induced long-term potentiation of synaptic connectivity7, an energy-demanding process that involves extensive biochemical and morphological adaptations at all levels of neuronal function8,9. There is also evidence for contributions of pre-existing developmental and other intrinsic programmes of individual neurons10, including the baseline expression of the transcriptional factor CREB11 and lineage of developmental origin12,13. Recent focus has also been on the role of the interneuronal perineuronal nets (PNNs) in the stabilization of memory circuits through tightened control of inhibitory inputs to dedicated neuronal assemblies14. Here we explored whether an overarching process could integrate stimulus-dependent and pre-existing mechanisms that underlie the commitment of neurons to memory-specific assemblies.










/cdn.vox-cdn.com/uploads/chorus_asset/file/23935561/acastro_STK103__04.jpg)
/cdn.vox-cdn.com/uploads/chorus_asset/file/24371483/236494_Mac_mini__2023__AKrales_0066.jpg)



