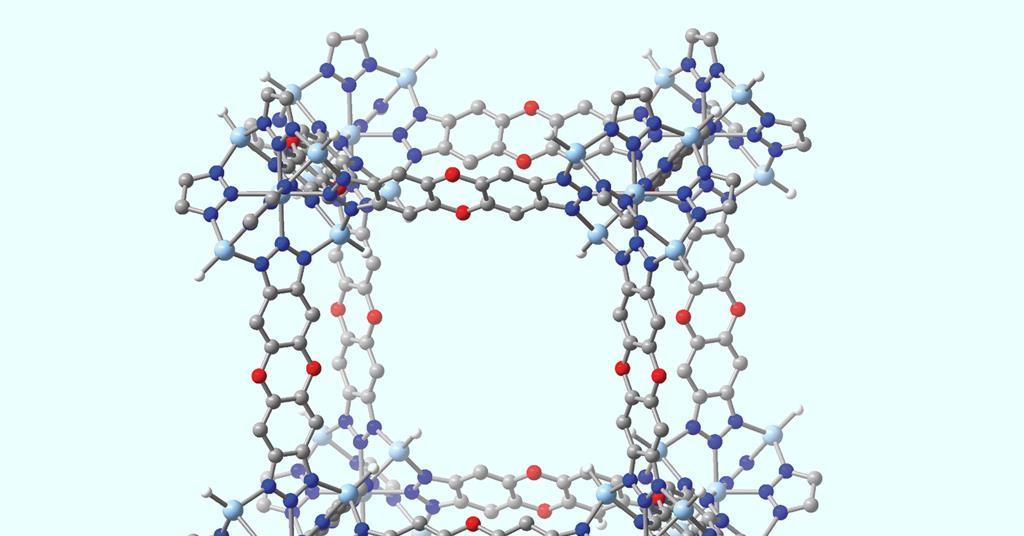
Metal–organic framework captures carbon dioxide at industrially relevant temperatures
A new metal–organic framework (MOF) structure functionalised with terminal zinc hydride sites can reversibly capture carbon dioxide at high temperatures.1 The material could enable easier, more energetically efficient capture of carbon from the exhaust gases of many industrial reactions. In future, it might also lower the temperature needed for some key processes.
The most mature technologies for capturing industrial carbon emissions are aqueous amines, but they only work at temperatures around 60°C or lower, whereas the exhaust gases from steel furnaces and cement kilns are vented at over 200°C. ‘You’re going to capture the carbon dioxide at low temperature and then release it at high temperature,’ says Jeffrey Long at University of California, Berkeley. ‘Temperature swings like that are usually inefficient.’
Instead, Long’s group has been experimenting with amine functionalisation of MOFs, which, as porous cage structures, are ideal for filtration. ‘Organic amines have a lot of degrees of freedom, and when you capture carbon dioxide two of [the amines] come together to make an ammonium carbamate ion pair – that locks them into place,’ notes Long. This means that there’s a large entropy change associated with capturing carbon dioxide with organic amines that disfavours the reaction at high temperatures, he explains.